ATX-101 is a cell penetrating peptide comprising the APIM motif as well as cell penetration and nuclear localization domains. In preclinical experiments, it was shown that ATX-101 rapidly penetrates cells and targets PCNA/APIM-containing protein complexes1.
By impairing interactions between PCNA with APIM-containing proteins, ATX-101 potentiates the action of several anti-cancer drugs. These include DNA damaging drugs, microtubulin targeting drugs, molecular targeted agents (e.g. p38, MAPK, EGFR inhibitors and others) and g-irradiation (>25 all together). In vivo, ATX-101 has shown proof-of-concept in blood and solid tumor animal models in combination with several clinically relevant drugs. Finally, ATX-101 shows strong anti-cancer action in myeloma patient-derived bone marrow samples ex vivo.
Because PCNA is an important scaffold protein involved in multiple cellular processes, targeting PCNA with ATX-101 (an APIM-peptide) leads to significant changes in gene expression, in the proteome/kinome status and in cellular metabolism. These changes include DNA repair and tolerance mechanisms, DNA damage signaling, MAPK/AKT/ERBB2/EGFR signaling and shifts in the apoptotic response. For example, see the scheme below for actions revealed in bladder cancer cells treated with cisplatin in combination with the APIM-peptide.
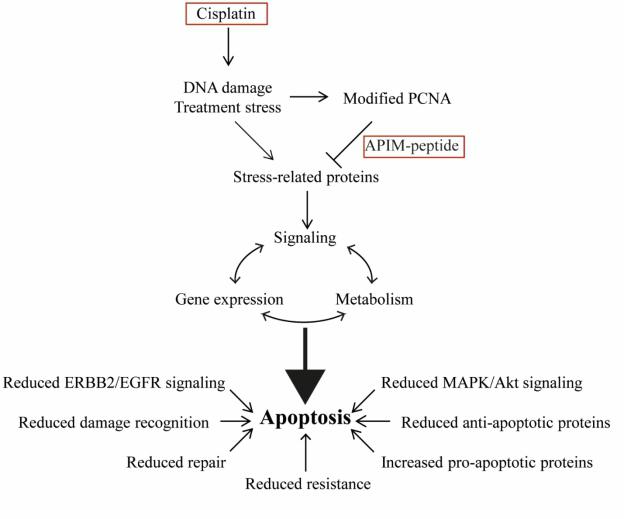
Figure 1. Mode of action for the increased apoptosis seen in bladder cancer cells in vivo (rats) and in vitro treated with APIM-peptide (ATX-101) in combination with cisplatin2. In addition, ATX-101 re-sensitizes cisplatin-resistant bladder cancer cells to treatment2.
In multiple myeloma cells, ATX-101 as a single agent induces apoptosis in the absence of cell division1. Single agent activity and strong apoptotic induction have also been observed in other cancer cells as well (e.g. glioblastoma)3.
ATX-101 has recently concluded a first-in-human, phase I clinical study. The study demonstrated a favorable safety profile of the compound. Furthermore, promising disease stabilization was observed in patients with advanced, progressive tumors lacking standard treatment options. The results of the phase I study of ATX-101 have been published4. A copy of the corresponding publication is available here.
Following conclusion of the phase I study, two additional studies have been initiated:
1. A phase Ib study in platinum-sensitive ovarian cancer, testing ATX-101 in combination with platinum-based chemotherapy. The study has recently been concluded and preliminary results have shown that the tested combination had a favorable safety profile and a high response rate. Based on these results, further clinical testing in this indication is warranted.
2. A phase II study in sarcoma (lipo-, and leiomyosarcoma) conducted as an Investigator Initiated Study by the Columbia University Irving Medical Center, evaluating ATX-101 as monotherapy. Preliminary data obtained in a small number of patients confirmed the therapeutic potential of ATX-101. This data, together with additional preclinical results obtained with an ATX-101 combination, support further phase II testing of the combination in lipo-, and leiomyosarcoma patients.
Multiple other development possibilities (e.g. with other chemotherapeutic or targeted agents) are also possible owing to the platform development possibilities of this intervention point.
For more information on the mechanism of ATX-101, see Intervention Point or Publications.
