APIM Therapeutics is targeting a novel therapeutic intervention point based on inhibition of protein protein interactions formed between:
- PCNA (Proliferating Cell Nuclear Antigen) and
- Proteins bearing the novel PCNA-interacting Peptide Motif, APIM (AlkB homolog 2 PCNA Interacting Motif).
PCNA is a scaffold protein essential for DNA replication and DNA repair. Lately, PCNA was also shown to be involved in epigenetics, apoptosis, immune evasion, glycolysis and cellular signaling. PCNA functions as a trimer interacting with multiple proteins via the IDCL (Inter-domain connector loop) and the underlying hydrophobic pocket to fulfill its functions.
Overall, two binding motifs are present in proteins interacting with PCNA at the hydrophobic pocket (see box in the scheme below)1,2.
PIP-box: Present in cell replication/housekeeping proteins binding to PCNA under normal conditions.
APIM: Present in “stress proteins” binding to PCNA during stress e.g. upon treatment with anti-cancer therapy.
PIP-box and APIM-containing proteins bind on overlapping binding sites on PCNA1-2. Their relative affinities to PCNA are regulated at multiple levels including multiple/different Post-Translational Modifications (PTMs) on PCNA.

Figure: Shows a front view of the PCNA ring (grey surface and ribbons) binding a PIP peptide (yellow sticks) from ZRANB3 (Zinc-finger, RAN-Binding domain containing 3) at the hydrophobic pocket in the same region where APIM binds. Figure reproduced without change in agreement with Creative Commons Attribution 4.0 International License provisions (see herein), from Sebesta M et al, Nat Commun. 2017 Jun 16;8:15847 (original Figure 6a in the publication).
Upon normal conditions, PCNA preferentially binds PIP-box-proteins e.g. normal housekeeping proteins responsible for cell division. However, upon DNA damage or cellular stress, e.g. cancer stress, PCNA is PTM-modified. This introduces an increase in the affinity of PCNA towards APIM, leading to a reduction of binding of housekeeping proteins and a recruitment of APIM-containing proteins involved in cellular defense mechanisms (shown in the Figure below).
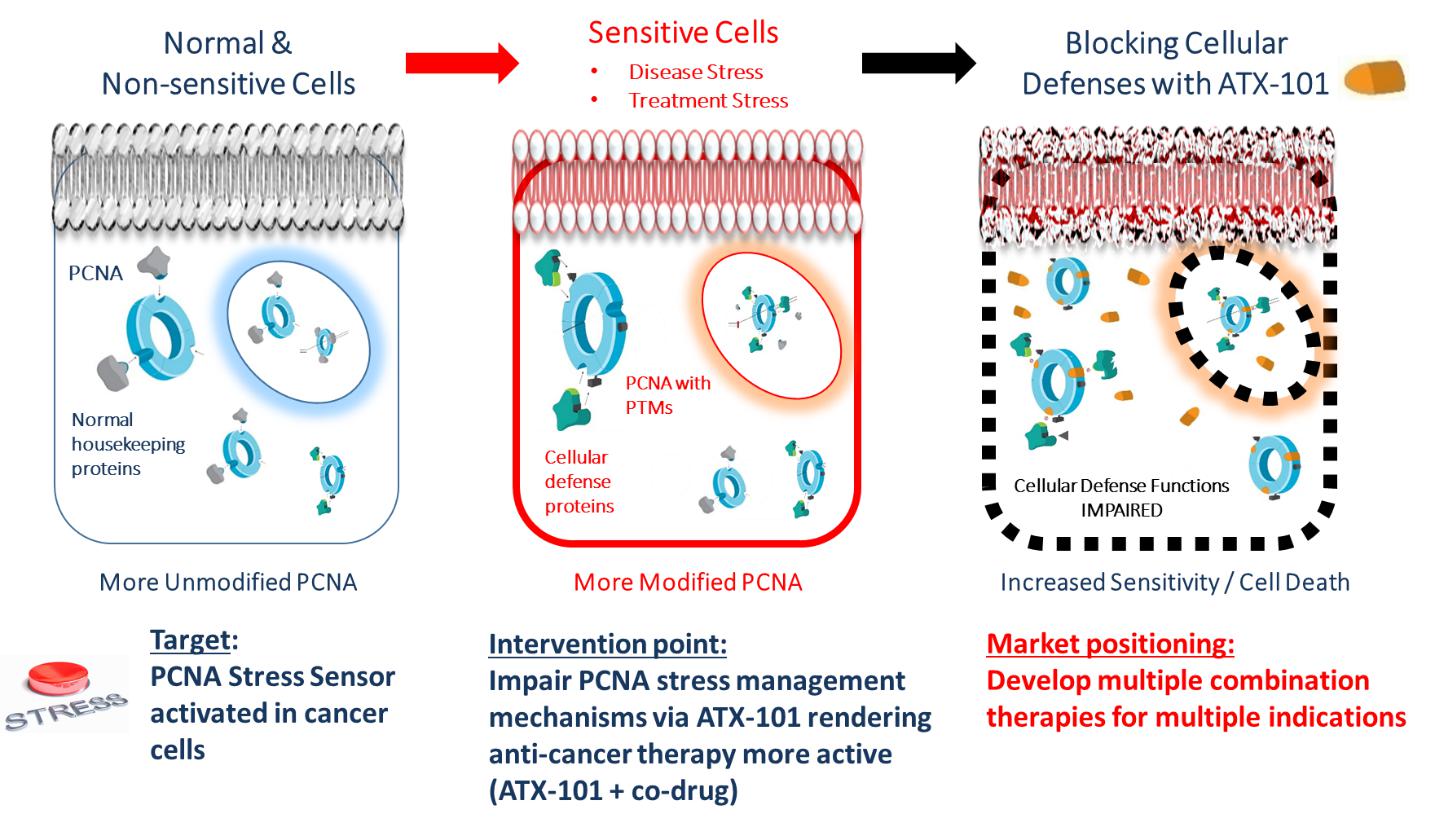
Targeting PCNA with ATX-101, a PCNA-APIM-Protein interaction blocking peptide, impairs PCNA scaffold functions and affects several cellular mechanisms involved in stress management. This leads to decreased survival and enhancement of the action of multiple anti-cancer drugs.
The aforementioned intervention point is relevant for multiple cancer indications and results in potentiation of >25 drugs (chemotherapeutics, targeted agents, radiation), as shown in several preclinical in vitro and in vivo models. In some solid tumor (e.g. glioblastoma) or blood cancer cells (e.g. multiple myeloma) that are known to have high levels of stress (for example replication stress), ATX-101 is capable of inducing apoptosis as a single agent3.
ATX-101 has recently concluded a first-in-human, phase I clinical study. The study demonstrated a favorable safety profile of the compound. Furthermore, promising disease stabilization was observed in patients with advanced, progressive tumors lacking standard treatment options. The results of the phase I study of ATX-101 have been published4. A copy of the corresponding publication is available here.
APIM Therapeutic’s current clinical positioning focuses on the development of multiple combination therapies for multiple indications. Based on available preclinical data showing increased efficacy of cisplatin-based therapies and re-sensitization of platinum-resistant cells to platinum induced by ATX-1015, we have conducted a phase Ib study in platinum sensitive ovarian cancer testing ATX-101 in combination with platinum-based chemotherapy. The study has recently been concluded; preliminary results have shown that the tested combination had a favorable safety profile and a high response rate. Additional combination development is also planned in glioblastoma in the near future (ATX-101 combined with temozolomide and radiotherapy).
Following promising preclinical results and data obtained during our phase I study, a phase II study in sarcoma (lipo-, and leiomyosarcoma) conducted as an Investigator Initiated Study has been initiated by the Columbia University Irving Medical Center. The study evaluated ATX-101 as a single agent therapy. Preliminary data obtained in a small number of patients confirmed the therapeutic potential of ATX-101. This data, together with additional preclinical results obtained with an ATX-101 combination, support further phase II testing of the combination in lipo-, and leiomyosarcoma patients.
For more information on this mechanism of action, see Publications.
