APIM Therapeutics is developing a series of proprietary Protein Protein Interaction (PPI) blocking peptides targeting complexes formed between PCNA1 (Proliferating Cell Nuclear Antigen) and proteins bearing a novel PCNA-interacting Peptide Motif called APIM2 (AlkB homolog 2 PCNA Interacting Motif). These compounds are particularly relevant for the treatment of hyper-proliferative disorders such as cancer.
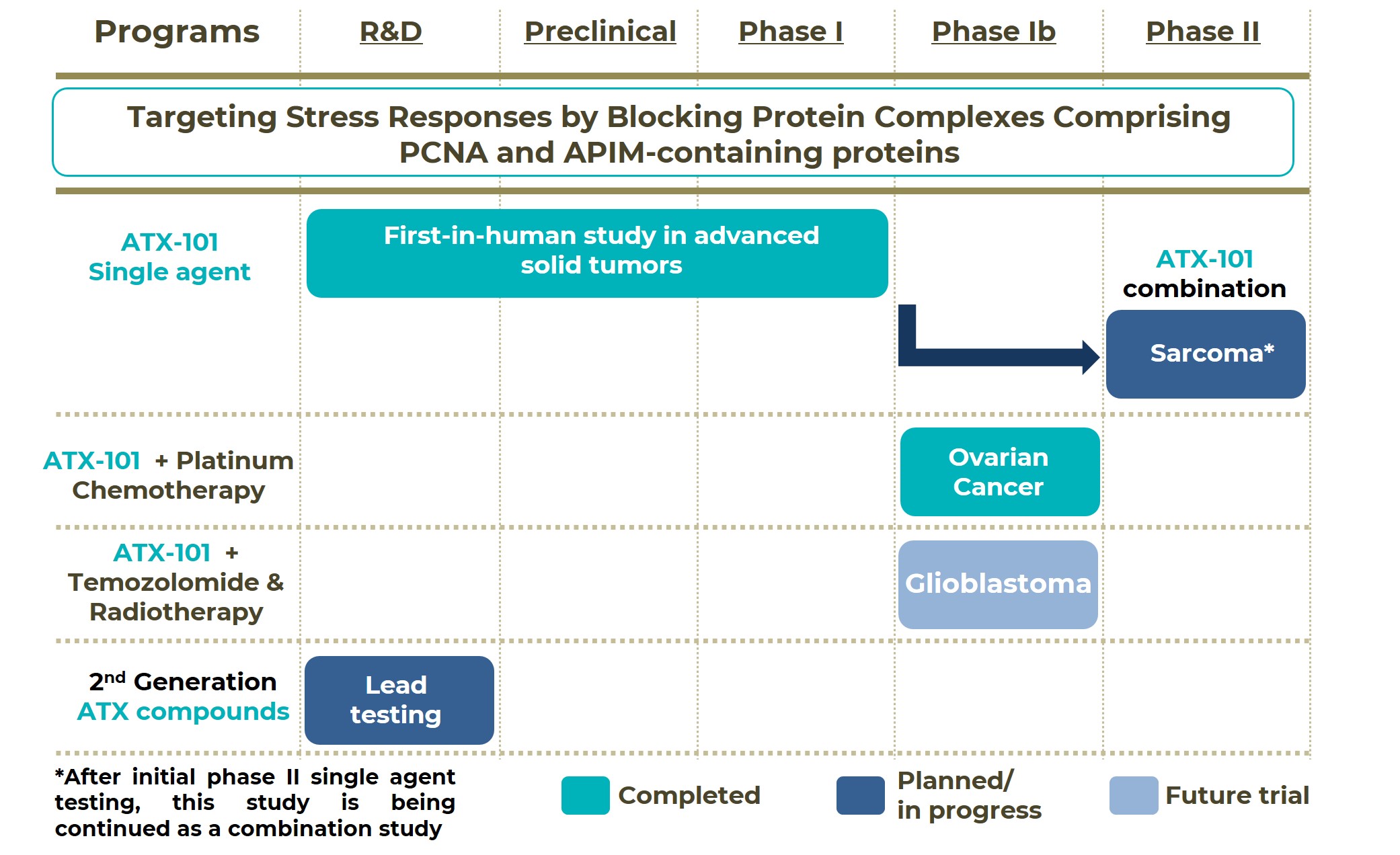
Our therapeutic candidate, ATX-101, is a first-in class PCNA/APIM PPI blocking compound currently being developed for the treatment of several cancer indications either alone or in combination with selected anti-cancer agents. A snapshot of the drug pipeline of APIM Therapeutics is presented below. The company has recently concluded a phase I, first-in-human study in patients with advanced solid tumors. Following conclusion of the phase I study, two additional studies have been initiated:
1. A phase Ib study in platinum-sensitive ovarian cancer conducted in Australia, testing ATX-101 in combination with platinum-based chemotherapy. The study has recently been concluded and preliminary results have shown that the tested combination had a favorable safety profile and a high response rate. Based on these results, further clinical testing in this indication is warranted.
2. A phase II study in sarcoma (lipo-, and leiomyosarcoma) conducted as an Investigator Initiated Study by the Columbia University Irving Medical Center, evaluating ATX-101 as monotherapy. Preliminary data obtained in a small number of patients confirmed the therapeutic potential of ATX-101. This data, together with additional preclinical results obtained with an ATX-101 combination, support further phase II testing of the combination in lipo-, and leiomyosarcoma patients.
Other indications/combinations are also of further interest for future applications and are currently being planned e.g. in glioblastoma. The company is also working on 2nd generation ATX molecules with improved properties; a few leads are currently being tested. A lead candidate is expected to be selected within 2025 and will subsequently enter preclinical development.
For more information on our pipeline, feel free to contact us directly.
